Fluorescent chromophores are compounds that can emit light under certain circumstances. They are useful to chemists as dyes or markers, and can be used for such applications as medical and biological imaging.
In a recent paper that appeared in the Journal of the American Chemical Society, Professor Victor Nemykin (Chemistry and Biochemistry, University of Minnesota Duluth) and his collaborators at the University of Akron (Akron, Ohio) discuss a new fluorescent chromophore, or fluorophore, called BOPHY. (BOPHY stands for bis(difluoroboron)1,2-bis(1H-pyrrol-2-yl)methylene)hydrazine.) This compound is related to a very successful class of fluorophores already in use, the boron dipyrromethene family of compounds. BOPHY is important because it can be produced with a simple two-step procedure, and it is highly fluorescent.
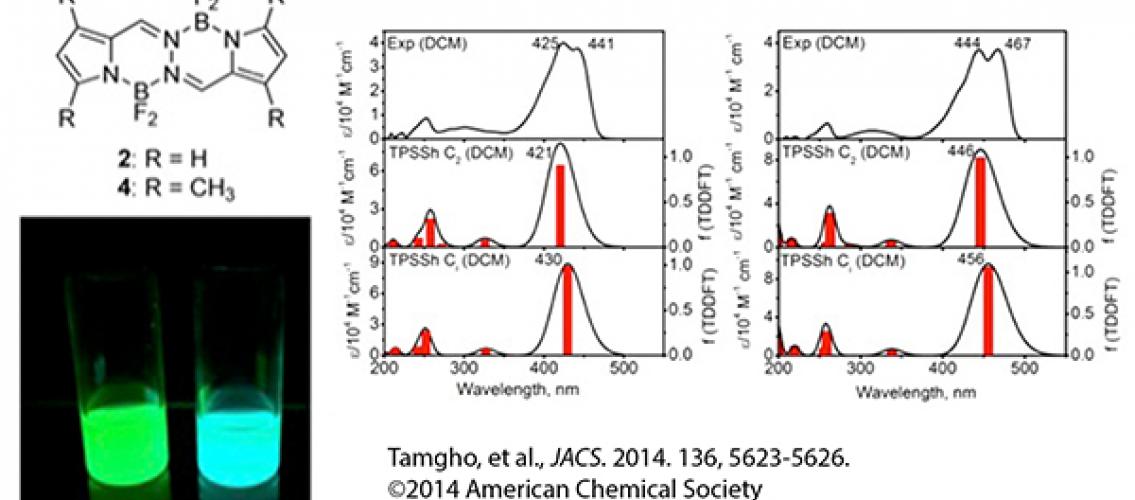
The researchers studied two compounds, the BOPHY chromophore and a tetrameythyl-substituted BOPHY analogue called Me4BOPHY. They investigated the absorption and emissions spectra of these compounds using density functional theory (DFT) and time-dependent DFT (TDDFT) calculations. The graphs on the right side of the figure above show the (top) experimental and (middle, bottom) TDDFT-predicted absorption spectra of (left) 2 and (right) 4 in dichloromethane, showing the excellent agreement between theory and experiment. The left of the figure shows a schematic of BOPHY (2) and Me4BOPHY (4) (top) and their fluorescence property.
This paper first appeared on the JACS website in April 2014. It was published in the April 16, 2014 print edition of the journal (Tamgho, Ingrid-Suzy, Abed Hasheminasab, James T. Engle, Victor N. Nemykin, and Christopher J. Ziegler. 2014. A new highly fluorescent and symmetric pyrrole-BF2 chromophore: BOPHY. Journal of the American Chemical Society 136 (15) (APR 16): 5623-6).
posted on August 6, 2014