Associate Professor Jeff Schwinefus, who is a faculty member at St. Olaf College in Northfield, Minnesota, is a physical chemist investigating the role of naturally occurring organic molecules in the hydration and stability of nucleic acids. He and his students perform experiments in the lab and also use molecule dynamics (MD) simulation techniques. This is interdisciplinary research that involves chemistry, biology, and physics.
The Schwinefus group’s research at MSI studies the cosolutes glycine betaine, proline, and urea, which all destabilize DNA and RNA secondary structures. The cosolutes interact with the nucleic acid surface area exposed during unfolding. The group is using lab work and MD simulations to understand this process.
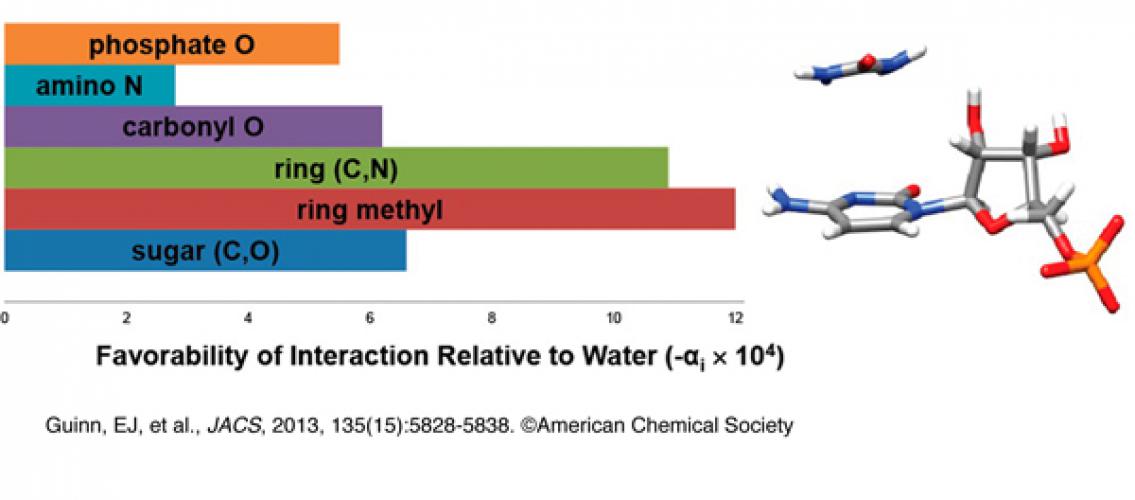
Professor Schwinefus, members of his research group, and colleagues at the University of Wisconsin-Madison, published a recent paper about their work in the Journal of the American Chemical Society (Guinn, Emily J., Jeffrey J. Schwinefus, Hyo Keun Cha, Joseph L. McDevitt, Wolf E. Merker, Ryan Ritzer, Gregory W. Muth, Samuel W. Engelsgjerd, Kathryn E. Mangold, Perry J. Thompson, Michael J. Kerins, and M. Thomas Record. 2013. Quantifying functional group interactions that determine urea effects on nucleic acid helix formation. Journal of the American Chemical Society 135 (15) (APR 17): 5828-38). This paper investigated urea as a destabilizing agent of the helical and folded conformations of nucleic acids and proteins as well as protein-nucleic acid complexes. Their results demonstrated that urea can be used as a quantitative probe of conformational changes in nucleic acid processes.
Image description: Interaction potentials quantifying interactions of urea with unit surface areas of nucleic acid functional groups (heterocyclic aromatic ring, ring methyl, carbonyl and phosphate O, amino N, sugar (C and O)); urea interacts favorably with all these groups. Image and description taken from Guinn, EJ, et al., 2013. JACS 135(15): 5828-38. © American Chemical Society
Posted on June 4, 2014.