Breast cancer is the second-leading cause of cancer death in women. Researchers are developing methods of targeting specific types of breast cancer, providing a personalized approach to treatment. One such method targets the Janus kinase(JAK)/signal transducer and activator of transcription (STAT) pathway, which causes tumors to develop. However, there has been little research into how this kind of treatment might affect other components of the tumor microenvironment.
MSI PIs from the Department of Laboratory Medicine and Pathology and members of MSI’s Research Informatics Solutions group, with group members and colleagues, recently published a paper in the journal Proceedings of the National Academy of Sciences of the United States of America that describes their research into JAK/STAT inhibitors and their effect on cells of the tumor microenvironment. The researchers demonstrated how JAK/STAT inhibitors can cause an increase in inflammation factors, which encourage tumor growth. They also showed that reducing the inflammatory factors can increase the effectiveness of JAK/STAT inhibitors. The paper can be found on the journal website (MSI PIs are in bold; MSI RIS staff are in italics): EA Irey, CM Lassiter, NJ Brady, P Chuntova, Y Wang, TP Knutson, C Henzler, TS Chaffee, RI Vogel, AC Nelson, MA Farrar, K Schwertfeger. 2019. JAK/STAT inhibition in macrophages promotes therapeutic resistance by inducing expression of protumorigenic factors. Proceedings of the National Academy of Sciences of the United States of America 116(25): 12442-12451. doi: 10.1073/pnas.1816410116.
Assistant Professor Andrew Nelson uses MSI for research concerning ovarian, endometrial, and breast cancer as well clinical evaluation of new diagnostic assays and pipelines. Professor Michael Farrar uses MSI for projects relating to signal transduction and lymphocyte development and their roles in leukemia. Associate Professor Kaylee Schwertfeger is studying how macrophages contribute to breast cancer. Professors Nelson, Farrar, and Schwertfeger are also members of the Masonic Cancer Center. Dr. Christine Henzler led the RIS Laboratory Medicine and Pathology group at the time of the research, and Dr. Todd Knutson is a bioinformatics analyst in that group.
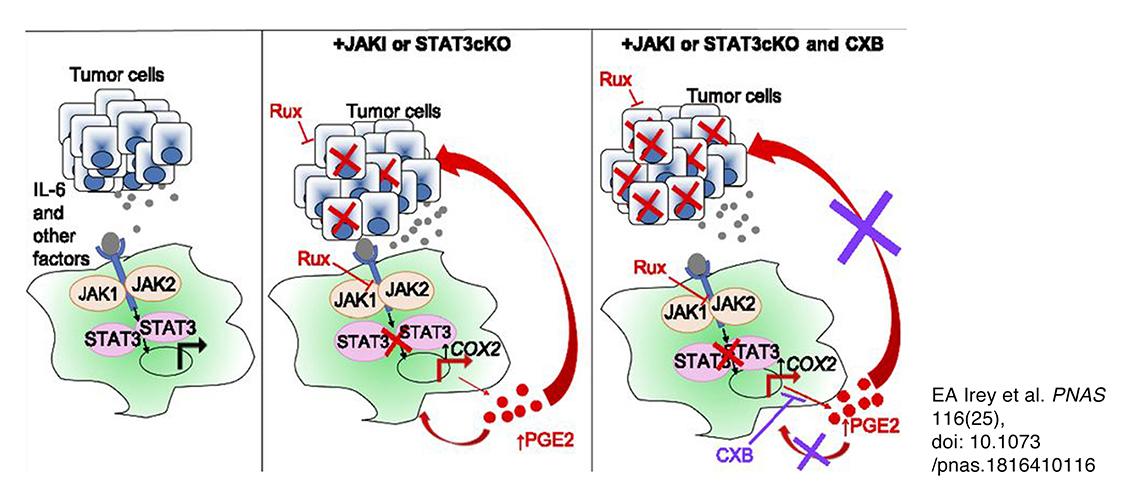
Image description: Graphical abstract illustrating the key concepts that tumor cell-derived factors, including IL-6 family cytokines, contribute to activation of STAT3 signaling in macrophages (Left). Inhibition of STAT3, either genetically or pharmacologically, leads to increased production of PGE2, which potentially acts via autocrine and/or paracrine mechanisms on tumor cells and macrophages to promote therapeutic resistance (Center). Targeting PGE2 production with celecoxib enhances responsiveness of tumor cells to ruxolitinib (Right). Image and description from EA Irey et al. PNAS 116(25), doi: 10.1073/pnas.1816410116.
posted on August 18, 2020