A major concern of medical treatment is that patients can develop infections, either from exposure to dangerous microbes while in the hospital, or because these organisms are introduced by medical devices, such as artificial joints, catheters, or artificial heart valves. These infections affect thousands of patients a year. The second most common cause of healthcare-related infections is Enterococcus faecalis, a bacterium that exists normally in the human digestive system, but can cause serious infection if introduced to other areas of the body.
Professor Gary Dunny (Microbiology and Immunology and BioTechnology Institute) and his lab are interested in the genetics, pathogenicity, and cell-cell signaling in the enterococci bacteria, with special emphasis on biofilm formation, antibiotic resistance, and virulence. Biofilms are communities of microorganisms that form on surfaces and are generally encased within an extracellular matrix produced by the attached microbes. When pathogens such as E. faecalis form biofilms, they are often highly resistant to both the host immune system and to antibiotic treatment and can cause chronic infections.
The Dunny group is using software packages available through MSI for analysis of next-generation sequencing data as part of their research into E. faecalis and biofilms. One recent area of interest was an investigation into how E. faecalis adapts as a pathogen in a mammalian host (Frank, Kristi L., Cristina Colomer-Winter, Suzanne M. Grindle, Jose A. Lemos, Patrick M. Schlievert, and Gary M. Dunny. 2014. Transcriptome analysis of Enterococcus faecalis during mammalian infection shows cells undergo adaptation and exist in a stringent response state. PLoS One 9, (12) (DEC 29), 10.1371/journal.pone.0115839.). Another project studied genes present in E. faecalis that affect biofilm formation and are factors in its virulence (Frank, Kristi L., Paschalis Vergidis, Cassandra L. Brinkman, Kerryl E. Greenwood Quaintance, Aaron M. T. Barnes, Jayawant N. Mandrekar, Patrick M. Schlievert, Gary M. Dunny, and Robin Patel. 2015. Evaluation of the Enterococcus faecalis biofilm-associated virulence factors ahrC and eep in rat foreign body osteomyelitis and in vitro biofilm-associated antimicrobial resistance. PLoS One 10, (6) (JUN 15), 10.1371/journal.pone.0130187.).
The Dunny group is also using MSI resources for high-throughput functional genomics studies to identify all critical gene products for biofilm growth and antibiotic resistance. Further information about their work can be found on the Department of Microbiology and Immunology and BioTechnology Institute websites.
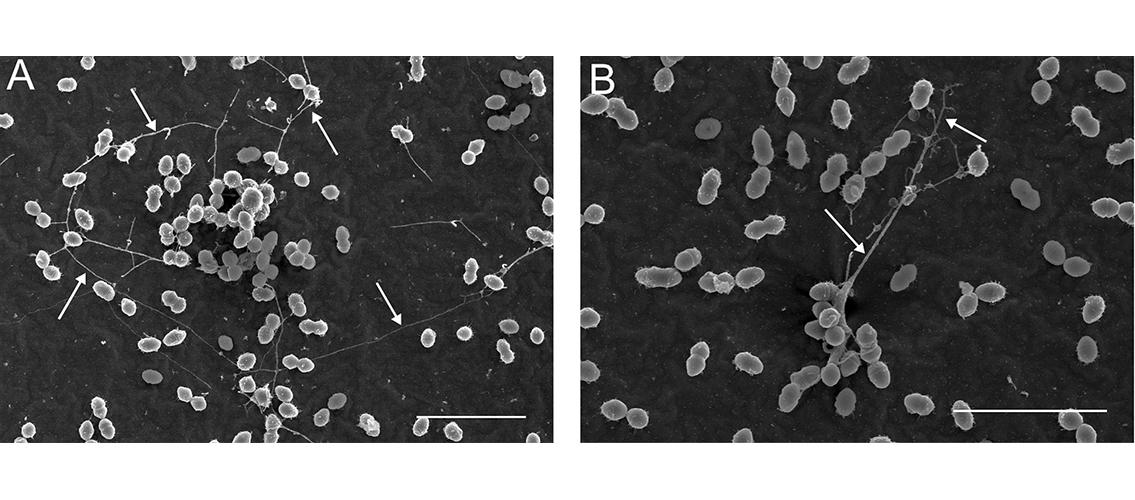
Image description: Long intercellular strands (yarn structures) visible in early (4 hour) E. faecalis biofilms (arrows). Bar, 5 mm. Image and description, Barnes, AMT, et al. 2012. mBio 3(4): e00193-12. DOI: 10.1128/mBio.00193.
published on October 28, 2015