Scientists who study the atmosphere have been interested in the behavior of sulfur compounds for many years. For example, sulfuric acid is a key player in the formation of atmospheric aerosol and, as such, plays a critical role in determining climate and the global energy budget. Further investigations into how sulfur combines with other atmospheric species continue to be important for both industrial and environmental purposes.
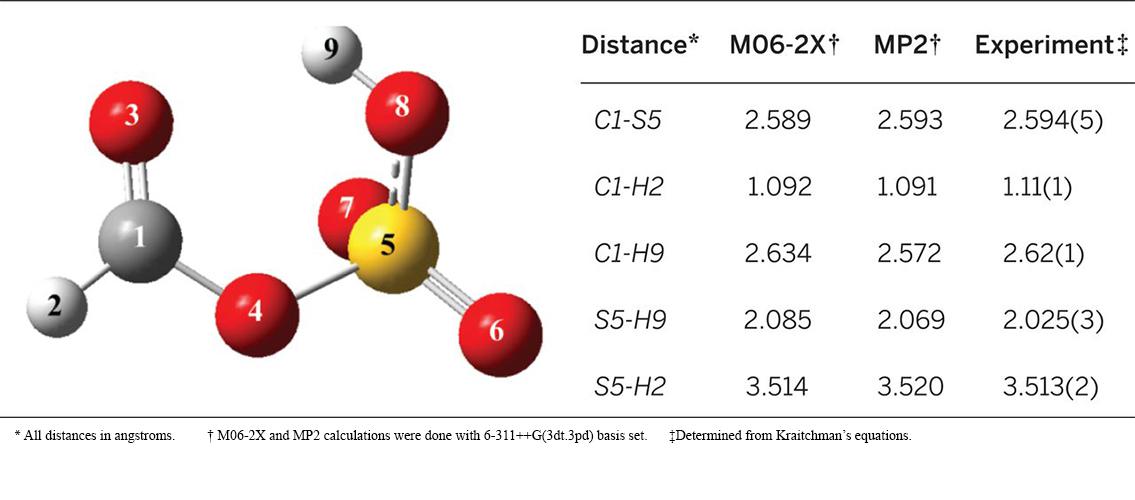
As part of their research of the formation of atmospheric aerosol particles, the group of Professor Kenneth Leopold (Chemistry) has been studying the chemistry of sulfur in the atmosphere. In a recent paper in the prestigious journal Science, Professor Leopold, post-doc Dr. Christopher Dewberry, and PhD student Becca Mackenzie described a new atmospheric species, formic sulfuric anhydride (FSA), which is produced from formic acid (HCOOH) and sulfur trioxide (SO3) under supersonic jet conditions. The authors created an experiment that mixed HCOOH, SO3, and water in a supersonically expanded mixture. They observed this new species in the mixture and used microwave spectroscopy, density functional theory, and ab initio calculations to determine its structure (see image) and probable formation mechanism. The entire paper can be found on the Science website: RB Mackenzie, CT Dewberry, and KR Leopold. 2015. Gas phase observation and microwave spectroscopic characterization of formic sulfuric anhydride. Science 349 (6243) (JUL 3): 58-61. See more about Professor Leopold’s research on the Ken Leopold Group website.
Image description: Comparison of experimental and theoretical intermolecular distances. Image and description RB Mackenzie, et al. 2015. Science 349: 58-61.
posted on October 8, 2015