The challenge of finding “green” energy resources to replace non-renewable fossil fuels is a topic of great interest for researchers. Chris Cramer (Distinguished McKnight Professor of Chemistry; MSI Fellow) and his research group and collaborators are among those who are using high-performance computational methods for their research in this area.
Plants have been using solar power to generate energy for millions of years. In photosynthesis, plants transform the sun’s rays into chemical energy that they can use. This process involves the oxidation of water in the plant. Researchers are interesting in creating artificial systems that can mimic this process. The method is known as water-oxidation catalysis, and, while it has been receiving a great deal of attention in recent years, a great deal of work still needs to be done to create a system that is economically practical for large-scale use.
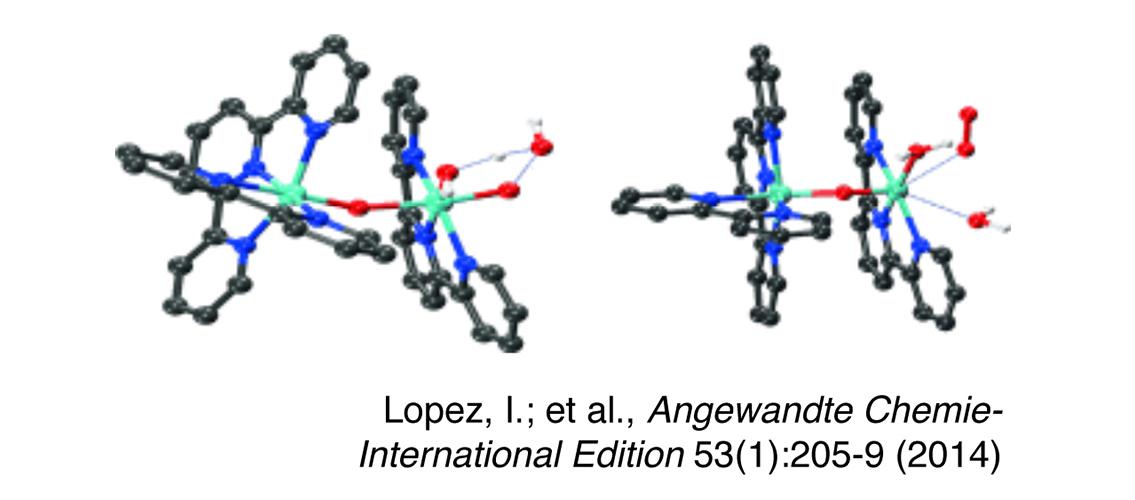
Professor Cramer and a former student and MSI researcher, Dr. Mehmed Ertem, in collaboration with other investigators at Brookhaven National Laboratory, the Catalonian Institute for Chemical Research, Berlin Technical University, and Yale University, recently published a paper in Angewandte Chemie that discusses a new water-oxidation catalyst (Lopez, Isidoro, Mehmed Z. Ertem, Somnath Maji, Jordi Benet-Buchholz, Anke Keidel, Uwe Kuhlmann, Peter Hildebrandt, Christopher J. Cramer, Victor S. Batista, and Antoni Llobet. 2014. A self-improved water-oxidation catalyst: Is one site really enough? Angewandte Chemie-International Edition 53 (1) (JAN 3): 205-9). The authors have demonstrated a dinuclear ruthenium (Ru) water-oxidation catalyst that is created from a mononuclear catalyst during the catalytic process. The dinuclear catalyst is rugged and powerful and does not decompose over time. The researchers used kinetic analysis and density functional theory computational studies to characterize this reaction.
Green energy is just one of the many topics under investigation by the Cramer group. They are developing computer models to study many areas of chemical, biological, and environmental interest and have a large number of publications. A sample is shown below:
- Angeles-Boza, Alfredo M., Mehmed Z. Ertem, Rupam Sarma, Christian H. Ibanez, Somnath Maji, Antoni Llobet, Christopher J. Cramer, and Justine P. Roth. 2014. Competitive oxygen-18 kinetic isotope effects expose O-O bond formation in water oxidation catalysis by monomeric and dimeric ruthenium complexes.Chemical Science 5 (3) (MAR): 1141-52.
- Gao, Jiali, B.J. Jankiewicz, J. Reece, H. Sheng, Christopher J. Cramer, J.J. Nash, H.I. Kenttämaa. 2014. On the factors that control the reactivity of meta-benzynes. Chemical Science. In press.
- Isley, William C., III, S. Zarra, R.K. Carlso, R.A. Bilbeisi, T.K. Ronson, J.R. Nitschke, Laura Gagliardi, Christopher J. Cramer. 2014. Predicting paramagnetic 1H NMR chemical shifts and state-energy separations in spin-crossover host-guest systems. Physical Chemistry Chemical Physics. In press.
- Lee, Kyuho, William C. Isley, III, Allison L. Dzubak, Pragya Verma, Samuel J. Stoneburner, Li-Chiang Lin, Joshua D. Howe, et al. [Christopher Cramer; Donald Truhlar; Laura Gagliardi] 2014. Design of a metal-organic framework with enhanced back bonding for separation of N2 and CH4. Journal of the American Chemical Society 136 (2) (JAN 15): 698-704.
- Meng, W., A.B. League, T.K. Ronson, J.K. Clegg, William C. Isley, D. Semrouni, Laura Gagliardi, Christopher J. Cramer, J.R. Nitschke. 2014. Empirical and theoretical insights into the structural features and host-guest chemistry of M8L4 tube architectures. Journal of the American Chemical Society 136, 3972.
- Hirahara, Masanari, Mehmed Z. Ertem, Manabu Komi, Hirosato Yamazaki, Christopher J. Cramer, and Masayuki Yagi. 2013. Mechanisms of photoisomerization and water-oxidation catalysis of mononuclear ruthenium(II) monoaquo complexes. Inorganic Chemistry 52 (11) (JUN 3): 6354-64.
- Marenich, Aleksandr V., Christopher J. Cramer, and Donald G. Truhlar. 2013. Generalized born solvation model SM12. Journal of Chemical Theory and Computation 9 (1) (JAN): 609-20.
- Marenich, Aleksandr V., Christopher J. Cramer, and Donald G. Truhlar. 2013. Reduced and quenched polarizabilities of interior atoms in molecules. Chemical Science 4 (6): 2349-56.
- Marenich, Aleksandr V., Christopher J. Cramer, and Donald G. Truhlar. 2013. Uniform treatment of solute-solvent dispersion in the ground and excited electronic states of the solute based on a solvation model with state-specific polarizability. Journal of Chemical Theory and Computation 9 (8) (AUG): 3649-59.
- McGrath, Matthew J., I-F Will Kuo, Brice F. W. Ngouana, Julius N. Ghogomu, Christopher J. Mundy, Aleksandr V. Marenich, Christopher J. Cramer, Donald G. Truhlar, and J. Ilja Siepmann. 2013. Calculation of the Gibbs free energy of solvation and dissociation of HCl in water via Monte Carlo simulations and continuum solvation models. Physical Chemistry Chemical Physics 15 (32): 13578-85.
- Miro, Pere, and Christopher J. Cramer. 2013. Water clusters to nanodrops: A tight-binding density functional study. Physical Chemistry Chemical Physics 15 (6): 1837-43.
- Semrouni, David, Isley,William C.,,III, Carine Clavaguera, Jean-Pierre Dognon, Christopher J. Cramer, and Laura Gagliardi. 2013. Ab initio extension of the AMOEBA polarizable force field to Fe2+. Journal of Chemical Theory and Computation 9 (7) (JUL): 3062-71.
- Suess, Alison M., Mehmed Z. Ertem, Christopher J. Cramer, and Shannon S. Stahl. 2013. Divergence between organometallic and single-electron-transfer mechanisms in copper(II)-mediated aerobic C-H oxidation. Journal of the American Chemical Society 135 (26) (JUL 3): 9797-804.
Professor Cramer is a long-time MSI Principal Investigator and Fellow of the Institute. He is also a member of the Chemical Theory Center, whose other members include several MSI Principal Investigators: Regents Professor Donald Truhlar, Professor Laura Gagliardi, Professor J. Ilja Siepmann, and Professor Jiali Gao. MSI interviewed Professor Cramer about his research in September 2012.
Image Description: Ball-and-stick representation of the optimized transition-state structures for O–O bond-formation (left) and O2-evolution steps (right) for the Ru catalyst. H atoms are shown only for the aqua and hydroxy ligands. Figure and description © Angewandte Chemie; Lopez, I., et al., Angewandte Chemie-International Edition 53(1):205-9 (2014)
posted on May 7, 2014