Plasmas are used in industrial processes in several important high-technology industries, such as the semiconductor industry. One application is the use of chemically reactive plasmas to deposit extremely thin films on other materials. So-called “dusty” plasmas contain particulates; these plasmas are of great interest to industry, as particulates can be a source of contamination during the manufacturing processes, especially when the particulates are in the nano scale.
The behavior of these dusty plasmas has been studied by researchers in the group of Regents Professor Donald Truhlar (Chemistry; MSI Fellow). The Truhlar group is involved in a wide-ranging research program that is in the forefront of computational chemistry, including the structure and reactivity of nanoparticles. The research uses quantum mechanical, and quantum statistical methods. Much of this work is done using codes that have been developed by the Truhlar group themselves; they also use commercially available codes.
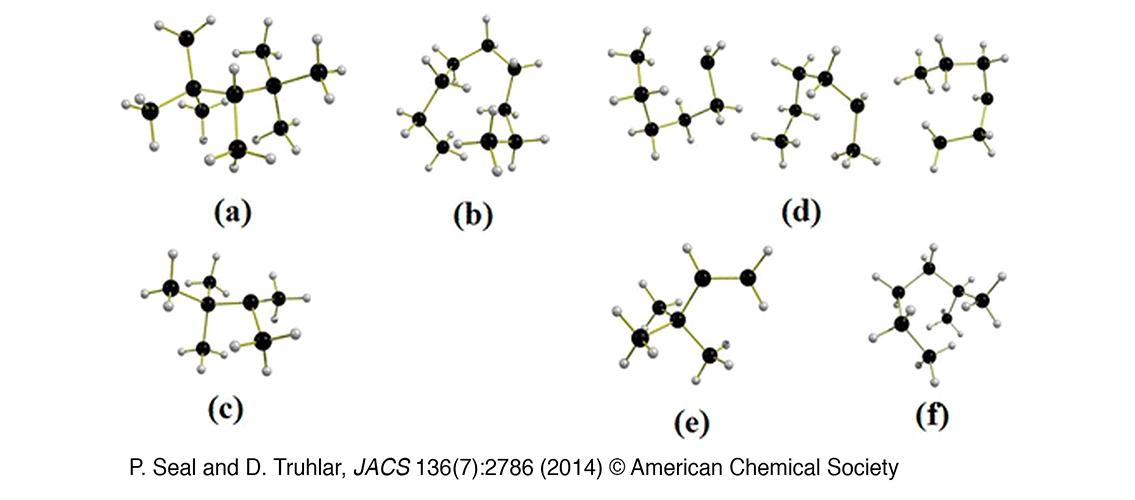
In a recent publication in the Journal of the American Chemical Society, post-doctoral researcher Dr. Prasenjit Seal and Professor Truhlar studied the thermodynamic properties of silane plasmas, which are widely used to grow silicon materials of use in electronics, photovoltaics, and photonics (Seal, Prasenjit and Donald Truhlar. 2014. Large entropic effects on the thermochemistry of silicon nanodusty plasma constituents. Journal of the American Chemical Society 136 (7): 2786-99). This research used a statistical mechanical approach combined with density functional theory. The authors studied five categories of silicon hydride clusters and radicals, including silanes, silyl radicals, and silenes, and showed the importance of multistructural effects.
The Truhlar group publishes a large number of papers every year and collaborates with many faculty researchers at the University of Minnesota and elsewhere. Some MSI Principal Investigators collaborating with Professor Truhlar include chemistry Professors Chris Cramer, Laura Gagliardi, and Jiali Gao, and medicinal chemistry Associate Professor Elizabeth Amin. Professor Truhlar is also a member of the Chemical Theory Center.
Other recent publications by the Truhlar group include:
- Klippenstein, Stephen J., Vijay S. Pande, and Donald G. Truhlar. 2014. Chemical kinetics and mechanisms of complex systems: A perspective on recent theoretical advances. Journal of the American Chemical Society 136 (2) (JAN 15): 528-46.
- Lee, Kyuho, William C. Isley, III, Allison L. Dzubak, Pragya Verma, Samuel J. Stoneburner, Li-Chiang Lin, Joshua D. Howe, et al. [Christopher Cramer; Donald Truhlar; Laura Gagliardi] 2014. Design of a metal-organic framework with enhanced back bonding for separation of N-2 and CH4. Journal of the American Chemical Society136 (2) (JAN 15): 698-704.
- Xu, Xuefei, Jingjing Zheng, Ke R. Yang, and Donald G. Truhlar. 2014. Photodissociation dynamics of phenol: Multistate trajectory simulations including tunneling. Journal of the American Chemical Society 136 (46) (NOV 19): 16378-86.
- Zheng, Jingjing, Ruben Meana-Paneda, and Donald G. Truhlar. 2014. Prediction of experimentally unavailable product branching ratios for biofuel combustion: The role of anharmonicity in the reaction of isobutanol with OH. Journal of the American Chemical Society136 (13) (APR 2): 5150-60.
- Han, Jaebeom, Michael J. M. Mazack, Peng Zhang, Donald G. Truhlar, and Jiali Gao. 2013. Quantum mechanical force field for water with explicit electronic polarization. Journal of Chemical Physics 139 (5) (AUG 7): 054503.
- Ho, Junming, Jingjing Zheng, Ruben Meana-Paneda, Donald G. Truhlar, Eun Jung Ko, G. Paul Savage, Craig M. Williams, Michelle L. Coote, and John Tsanaktsidis. 2013. Chloroform as a hydrogen atom donor in Barton reductive decarboxylation reactions. Journal of Organic Chemistry 78 (13) (JUL 5): 6677-87.
- Jalan, Amrit, Ionut M. Alecu, Ruben Meana-Paneda, Jorge Aguilera-Iparraguirre, Ke R. Yang, Shamel S. Merchant, Donald G. Truhlar, and William H. Green. 2013. New pathways for formation of acids and carbonyl products in low-temperature oxidation: The Korcek decomposition of gamma-ketohydroperoxides. Journal of the American Chemical Society 135 (30) (JUL 31): 11100-14.
- Kurbanov, Elbek K., Hannah R. Leverentz, Donald G. Truhlar, and Elizabeth A. Amin. 2013. Analysis of the errors in the electrostatically embedded many-body expansion of the energy and the correlation energy for Zn and Cd coordination complexes with five and six ligands and use of the analysis to develop a generally successful fragmentation strategy. Journal of Chemical Theory and Computation 9 (6) (JUN): 2617-28.
- Marenich, Aleksandr V., Christopher J. Cramer, and Donald G. Truhlar. 2013. Uniform treatment of solute-solvent dispersion in the ground and excited electronic states of the solute based on a solvation model with state-specific polarizability. Journal of Chemical Theory and Computation 9 (8) (AUG): 3649-59.
- Maurice, Remi, Pragya Verma, Joseph M. Zadrozny, Sijie Luo, Joshua Borycz, Jeffrey R. Long, Donald G. Truhlar, and Laura Gagliardi. 2013. Single-ion magnetic anisotropy and isotropic magnetic couplings in the metal-organic framework Fe-2(dobdc). Inorganic Chemistry 52 (16) (AUG 19): 9379-89.
Image description: M06-L/MG3S optimized global-minimum-energy structures of the largest representatives for each of the classes of systems considered in the paper: (a) fully branched silanes, Si10H22-FB; (b) unbranched silanes, n-Si9H20; (c) fully branched silyl radicals, Si7H15-FB; (d) unbranched silyl radicals, types I, II, and III, Si6H13; (e) fully branched silenes, Si6H12-FB; and (f) iso-Si7H16. Si atoms are black, and H atoms are light gray. (P. Seal and D. Truhlar, JACS 136(7):2786 (2014) © American Chemical Society)
posted on January 21, 2015
See all Research Spotlights.