MSI PIs Wei Zhang (research associate professor, Diagnostic and Biological Sciences) and Fang Li (associate professor, Veterinary and Biomedical Sciences) have published a new paper that describes some of their continuing research into the structure of coronaviruses. These are a large group of viruses that includes such deadly diseases as SARS and MERS. Coronaviruses have four forms, known as α-, β-, γ-, and δ-coronavirus, which affect different hosts. For example, β-coronaviruses affect only mammals, while the δ form affects both birds and mammals.
The coronavirus structure includes a feature called a “spike protein,” which allows the virus to attach to the host’s cells. The spike proteins of α- and β-coronavirus have been well studied. The spike protein of the δ-coronavirus, however, is described for the first time in this paper. The researchers used cryo-electron microscopy, a fast-developing technology in which protein molecules are studied under ultra-cold temperatures with an electron microscope. This technology was used to determine the structure of the spike protein of porcine δ-coronavirus (PdCoV), a lethal virus infecting pigs, elucidating how PdCoV infects pigs cells and evades the host immune system. This is the first atomic-resolution cryo-electron microscopic study from the state of Minnesota, and is a milestone in the structural biology field at the University of Minnesota.
The paper was published in late 2017 on the website of the Journal of Virology: J Shang, Y Zhang, Y Yang, Q Geng,W Tai, L Du, Y Zhou, W Zhang, F Li. 2018. Cryo-Electronic Microscopy Structure of Porcine Deltacoronavirus Spike Protein in the Prefusion State. Journal of Virology 92 (4): e01556-17. doi: 10.1128/JVI.01556-17.
The article was selected by the editors to be the journal spotlight, which is an article of significant interest in the issue.
Professor Zhang’s research using MSI resources involves analysis of cryo-electron microscopy images of bacterial phages, enveloped alphaviruses, and retroviruses. Professor Li uses MSI resources to support studies of the structural and molecular basis of diseases including viral infection, cancer, and abnormal blood pressure. Research into the coronavirus structure was previously featured on the MSI website in March 2017: Structural Comparisons of Two Receptors for Mouse Coronavirus. In further work by these researchers, a new paper describing the cryo-EM structure of a chicken coronavirus spike protein was published on the website of the journal PLoS Pathogens in late April 2018: J Shang, Y Zheng, Y Yang, C Liu, Q Geng, C Luo, W Zhang, F Li. 2018. Cryo-EM Structure of Infectious Bronchitis Coronavirus Spike Protein Reveals Structural and Functional Evolution of Coronavirus Spike Proteins. PLoS Pathogens 14 (4). doi: 10.1371/journal.ppat.1007009.
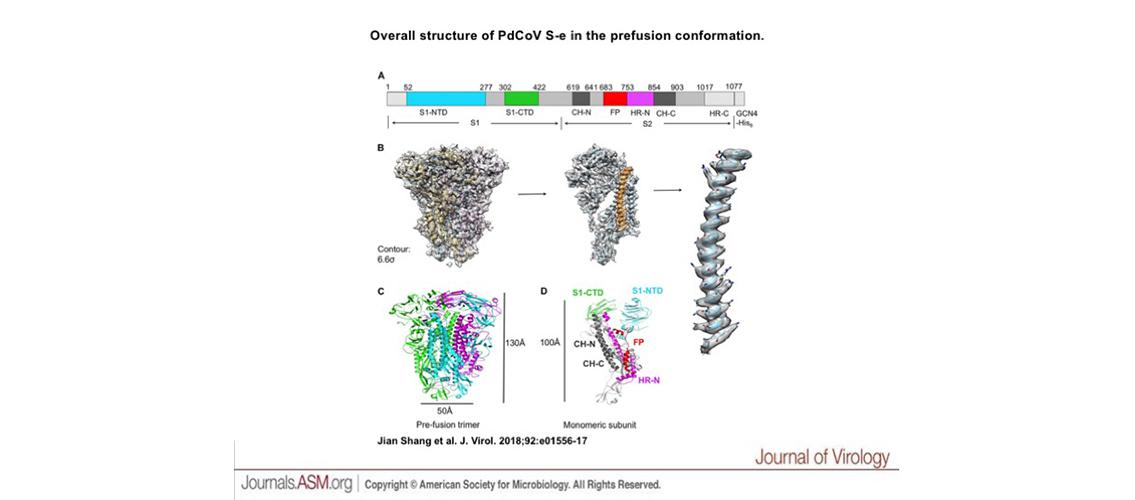
Image Description: Overall structure of PdCoV S-e in the prefusion conformation. (A) Schematic drawing of PdCoV S-e (spike ectodomain). S1, receptor-binding subunit. S2, membrane fusion subunit. GCN4-His6, GCN4 trimerization tag followed by His6 tag. S1-NTD, N-terminal domain of S1. S1-CTD, C-terminal domain of S1. CH-N and CH-C, central helices N and C. FP, fusion peptide. HR-N and HR-C, heptad repeats N and C. Residues in shaded regions (N terminus, GCN4 tag, and His6 tag) were not traced in the structure. (B) Cryo-EM maps of PdCoV S-e with atomic model fitted in. The maps have a contour of 6.6 σ. (C) Cryo-EM structure of prefusion PdCoV S-e. Each of the monomeric subunits is colored differently. (D) Structure of a monomeric subunit in the prefusion conformation. The structural elements are colored in the same way as those in panel A. Image and description, J Shang et al., J Virol. 92:e01556-17 (2018). © American Society for Microbiology.
posted on June 15, 2018