Adsorbent or catalytic materials called zeolites are used in the chemical and petrochemical industries to separate individual molecules based on size and shape. Building nanosheets of zeolites to make thin films for membrane applications has been a time-consuming, multi-step process. However, MSI researchers and their colleagues have developed a new, one-step method of creating zeolite nanosheets. Their work was published online in March in the journal Nature. MSI PIs involved in this research include lead researcher Michael Tsapatsis (Chemical Engineering and Materials Science); K. Andre Mkhoyan (Chemical Engineering and Materials Science); and J. Ilja Siepmann (Chemistry).
The new process involves a nanocrystal “seed” that grows and multiplies, eventually creating a sheet with uniform thickness. A key feature of this crystal growth is that it occurs in only two dimensions. The nanosheets created by the researchers measure 5 nm in thickness and can placed on a substrate to form a molecular sieve membrane. The researchers used MSI for some of the theoretical calculations for this process and for understanding membrane structure and performance.
The paper can be found on the Nature website (MSI users are identified with an asterisk (*)): Mi Young Jeon, Donghun Kim, Prashant Kumar*, Pyung Soo Lee, Neel Rangnekar, Peng Bai*, Meera Shete, Bahman Elyassi, Han Seung Lee, Katabathini Narasimharao, Sulaiman Nasir Basahel, Shaeel Al-Thabaiti, Wenqian Xu, Hong Je Cho, Evgenii O. Fetisov*, Raghuram Thyagarajan*, Robert F. DeJaco*, Wei Fan, K. Andre Mkhoyan*, J. Ilja Siepmann*, Michael Tsapatsis*. 2017. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 543, 690–694. doi:10.1038/nature21421.
The College of Science and Engineering also published a story about this paper, which contains a link to a video showing how the nanosheet forms: Researchers develop groundbreaking process for creating ultra-selective separation membranes.
Professor Tsapatsis and his research group use MSI for computational modeling that supports their studies of zeolites, especially zeolite nanosheets. Professor Mkhoyan performs computations and simulations to understand electron microscopy images and diffraction data. Professor Siepmann and his group develop and use computational-chemistry tools on the supercomputers in support of projects that investigate a variety of complex chemical systems and processes including adsorption and diffusion in zeolites.
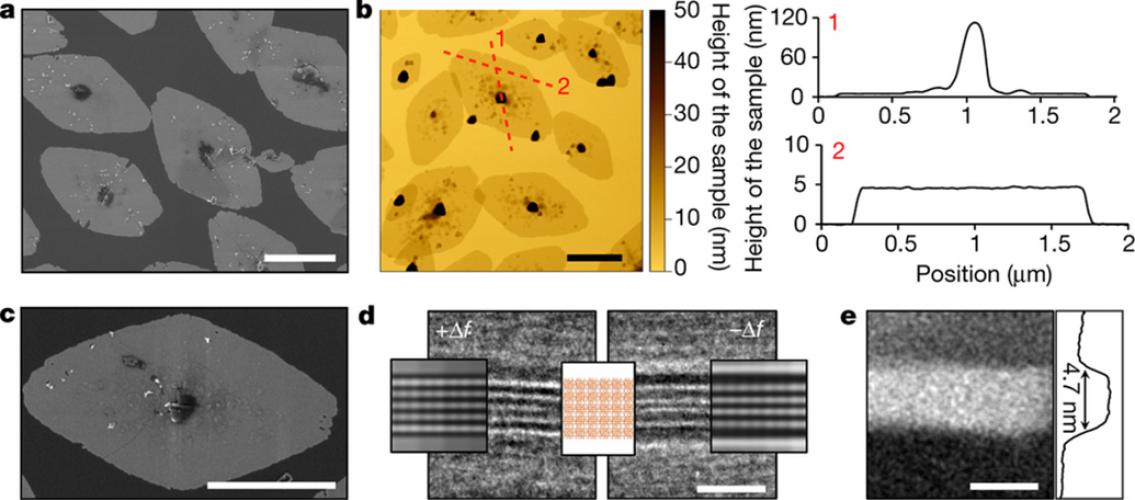
Image description: Characterization of the MFI nanosheet. a, SEM image for MFI nanosheets. Average sizes are about 2.0 μm and 1.2 μm along the.a- and c-axes, respectively. b. AFM height image and height profiles for the MFI nanosheets. The height profiles are extracted along the red dashed lines. The seed crystal at the centre is about 100 nm across, and the nanosheet exhibits a very uniform thickness of 4.6 ± 0.5 nm. c. High-magnification SEM image for the MFI nanosheet. The surface roughness of the nanosheets is due to the additional layer that forms on the top of the primary nanosheet. d. Underfocus (+∆f) and overfocus (−∆f) BF-TEM image with overlaid multislice simulations of a 5-pentasil-chain-thick MFI nanosheet section along the b-axis. White lines in the underfocus image and black lines in the overfocus image correspond to pentasil layers in the overlaid crystal structure model. The spacing between the black and white lines is dependent on TEM imaging conditions (defocus) and is not representative of the actual bond distances between the pentasil layers. e. HAADF-STEM image of the nanosheet (bright area) shown in d with a corresponding average intensity scan confirming the thickness of the MFI nanosheet to be 4.7 nm along the b axis. Scale bars in a, b and c are 1 μm and in d and e are 5 nm. Image and description, M.Y. Jeon et al. Nature 543:690–694 (2017), doi:10.1038/nature21421.
posted on June 1, 2017