The research group of Professor Steven Kass (MSI Fellow, Chemistry) uses MSI resources in their investigations into hydrogen bond networks. The goal of this research is to understand enzyme catalysis and develop hydrogen bond catalysts, novel acids and bases, and molecular anion receptors. By mimicking nature, the researchers have found that hydrogen bond arrays stabilize charged centers to a greater degree than was previously recognized and that flexible alcohols can serve as anion receptors. The Kass group has published results of their research in several articles that appeared in the Journal of the American Chemical Society during 2012 and 2011:
- “Three Hydrogen Bond Donor Catalysts: Oxyanion Hole Mimics and Transition State Analogues,” EV Beletskiy, J Schmidt, XB Wang, and SR Kass, JACS:134(45):18534, DOI:10.1021/ja3085862 (2012)
- “Characterization of a Saturated and Flexible Aliphatic Polyol Anion Receptor,” A Shokri, J Schmidt, XB Wang, and SR Kass, JACS 134(41):16944, DOI:10.1021/ja3075456 (2012)
- “Effect of Hydrogen Bonds on pKa Values: Importance of Networking,” A Shokri, A Abedin, A Fattahi, and SR Kass, JACS 134(25):10646, DOI:10.1021/ja3037349 (2012)
- "Hydrogen Bonded Arrays: The Power of Multiple Hydrogen Bonds," A Shokri, J Schmidt, XB Wang, and SR Kass, JACS 134(4):2094, DOI:10.1021/ja2081907 (2011)
The group is modeling a number of polyols and comparing their thermodynamic properties and binding properties with different substrates. This work benefits from the use of the supercomputers because the molecules are large and flexible, which makes it computationally expensive to examine them. The catalyst-substrate complexes also involve very time-consuming calculations. Current research in 2013 involves modeling infrared spectra and electron binding energies of a number of deprotonated polyols (the conjugate bases of Bronsted acids) and polyol-substrate complexes (bound hydrogen bond catalysts). This work complements experimental efforts that entail synthesizing novel “green” acids, hydrogen bond catalysts, and hosts for molecular recognition, as well as probing their reactivities and properties (e.g. pKa’s), and examining their binding via mass spectrometry experiments (e.g. electron affinity measurements and IR spectra determinations of bound catalysts).
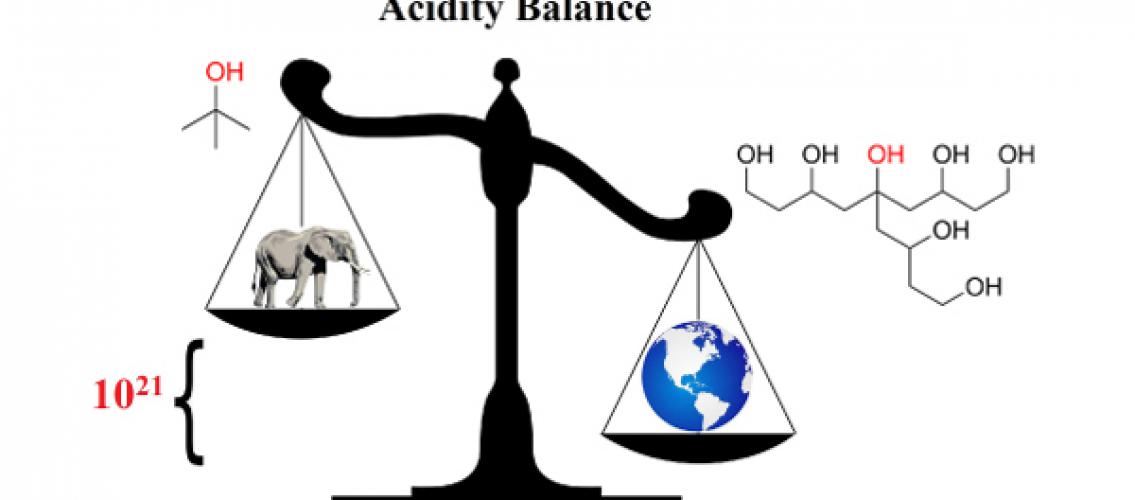
Description of image: The difference in the acidity of the two compounds shown is 21 orders of magnitude (i.e., 1021, a very large number!), due to the hydrogen bond network in the larger species. To put this number into context, it corresponds to the weight of an elephant (more precisely, a male African elephant) versus that of the entire world. That is, the ratio of the weight of the world to that of an elephant is the same as the ratio of the acidity difference of the polyol to the simple alcohol.